Understanding how well a drug is absorbed in the human body is a fundamental part of drug discovery. For orally administered medicines, the Human Intestinal Absorption (HIA) rate determines how much of the compound reaches systemic circulation — directly influencing bioavailability, efficacy, and dosage optimization.
To support faster, more data-driven research, SMAG has introduced its Human Intestinal Absorption (HIA) Prediction Model, a new addition to the TOPIA ADMET Suite (Absorption, Distribution, Metabolism, Excretion, and Toxicity). This model offers a reliable in silico method for evaluating absorption potential early in the R&D process, helping teams prioritize compounds with better pharmacokinetic properties while reducing the need for resource-intensive in vivo studies.
Why HIA Prediction Matters
Human Intestinal Absorption (HIA) represents the fraction of an orally administered drug that successfully enters systemic circulation after passing through the gastrointestinal tract. It’s one of the most critical determinants of oral drug effectiveness.
A drug's absorption potential affects:
- Bioavailability How much of the drug reaches its target site.
- Dose optimization Determining the appropriate dosage for therapeutic effectiveness.
- Candidate selection Prioritizing compounds with suitable absorption characteristics.
Traditional in vivo absorption studies can be costly, time-consuming, and resource-heavy. Reliable computational prediction offers a faster alternative that supports early-stage screening and decision-making — ensuring resources are focused on the most promising candidates.
What SMAG Built
The SMAG HIA Prediction Model combines advanced cheminformatics with robust statistical validation to deliver accurate and interpretable results suitable for high-throughput screening.
Model Highlights:
- Model Architecture: ExtraTrees classifier trained using Morgan fingerprints derived from SMILES input.
- Independent validation: Tested against external datasets to confirm real-world performance.
- Evaluation Strategy: Rigorously validated on training, test, and independent external datasets, ensuring broad applicability.
- Metrics Used: Accuracy, ROC-AUC, F1-score, Matthews Correlation Coefficient (MCC), and Balanced Accuracy (BACC).
- Usability: Designed for fast, interpretable, and user-friendly operation, making it ideal for early-stage R&D teams.
This design supports both scientific precision and practical use — allowing researchers to predict HIA efficiently without complex computational setups.
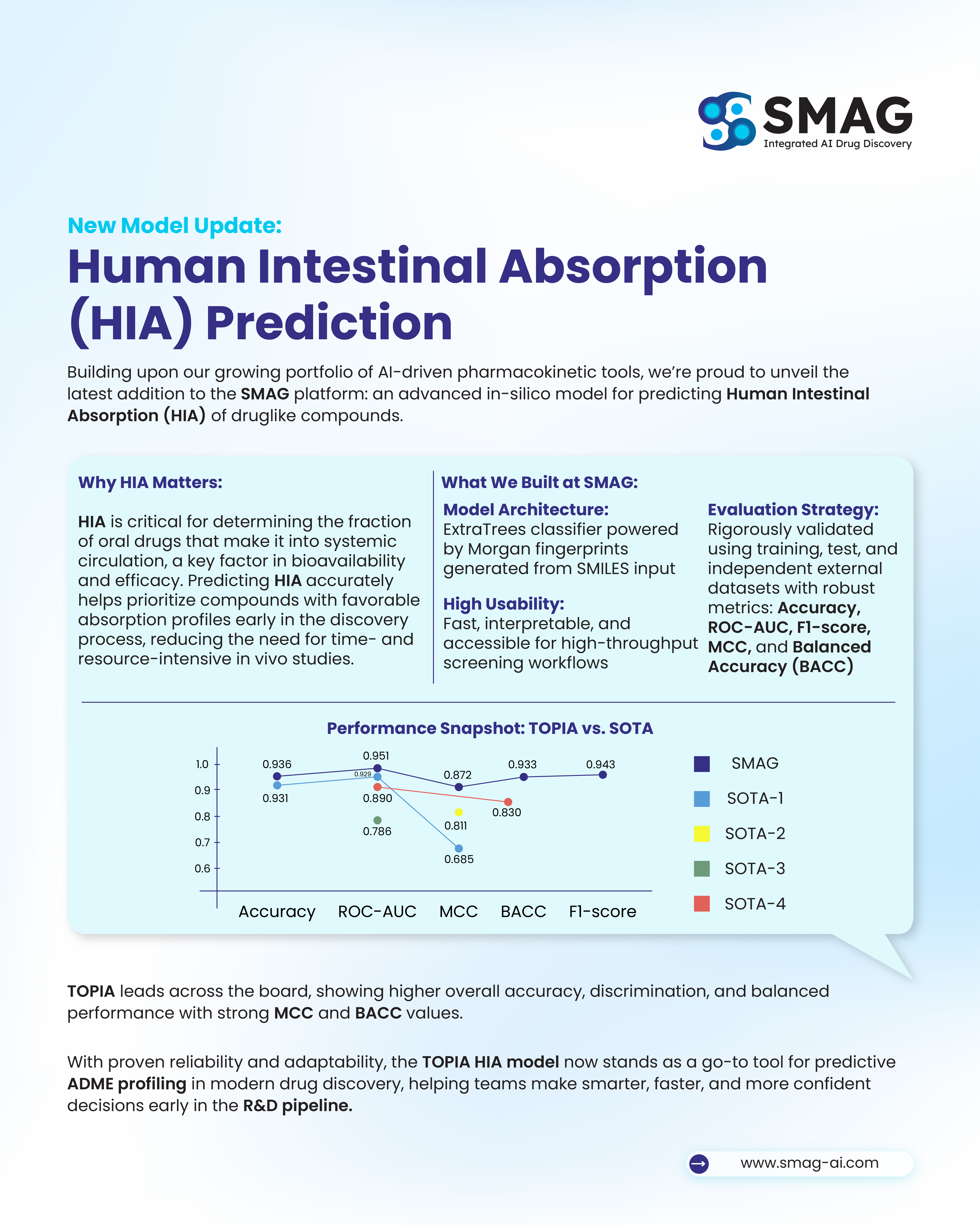
Model Performance
Extensive benchmarking confirms the SMAG HIA model delivers top-tier performance compared to state-of-the-art (SOTA) approaches.
| Model | Accuracy(%) | ROC-AUC | MCC | BACC | F1-score |
|---|---|---|---|---|---|
| SMAG | 0.936 | 0.951 | 0.872 | 0.933 | 0.943 |
| SOTA 1 | 0.931 | 0.929 | 0.685 | - | - |
| SOTA 2 | - | - | 0.811 | - | - |
| SOTA 3 | - | 0.786 | - | - | - |
| SOTA 4 | - | 0.890 | - | 0.830 | - |
Key Insights
- Reliability Under Imbalance:Achieves strong predictive stability with an MCC of 0.872, offering a more dependable measure than accuracy in imbalanced datasets.
- Robust Accuracy:Delivers 93.6% accuracy, reflecting overall consistency while complemented by balance-focused metrics.
- Superior ROC-AUC: A 0.951 ROC-AUC demonstrates exceptional discrimination capability across classes and thresholds.
- Balanced Generalization: A High Balanced Accuracy (0.933) confirms the model’s ability to perform reliably across both majority and minority classes.
The TOPIA HIA model consistently outperforms existing benchmarks, positioning it as a trusted predictive ADME tool for modern drug discovery.
Applications in Drug Discovery
The SMAG HIA model supports various stages of preclinical and early-stage research, helping scientists make informed decisions with data-backed confidence.
Key Use Cases
- Early compound screening - Identify molecules with favorable absorption profiles.
- Pharmacokinetic modeling -Integrate HIA predictions with other ADME parameters for comprehensive profiling.
- Candidate prioritization -Focus laboratory efforts on compounds with strong absorption potential.
- Resource optimization - Reduce reliance on time-consuming in vivo absorption studies.
By integrating this model into their workflow, researchers can accelerate discovery timelines and improve overall success rates in oral drug development.
Looking Ahead
The Human Intestinal Absorption Prediction Model is a reflection of SMAG's ongoing commitment to enhancing data-driven pharmacokinetics. As part of the TOPIA ADMET suite, it joins a growing set of validated predictive models — including permeability, distribution, and cytotoxicity — that together form a robust computational foundation for drug discovery.
By combining high accuracy, interpretability, and accessibility, the HIA model enables research teams to make smarter, faster, and more confident decisions at every stage of development.
Frequently Asked Questions (FAQs)
Conclusion
The SMAG Human Intestinal Absorption (HIA) Prediction Model marks another milestone in advancing computational pharmacokinetics. With high predictive accuracy and strong validation performance, it provides an efficient, reliable solution for early-stage absorption screening — helping researchers bring safe, effective therapies to patients faster.
To explore SMAG’s models or request a demo, visit smag-ai.com.